Abstract
Introduction: Cardiovascular (CV) toxicity has been reported with use of proteasome inhibitors (PI) for multiple myeloma (MM). However, detailed descriptions of the frequency and severity of cardiovascular adverse events (CVAE) and risk factors associated with CVAEs are lacking. We conducted a prospective, observational, multi-institutional study to define CV risk factors and outcomes in MM patients receiving bortezomib or carfilzomib.
Methods: Patients with relapsed MM who were starting therapy with a regimen containing either carfilzomib (Car)- or bortezomib (Bor)- underwent a detailed baseline CV assessment including: assessment of traditional CV risk factors (CVRF; family history, hypertension (HTN), hyperlipidemia, diabetes and tobacco use), cardiac biomarkers [troponin I and T, brain natriuretic peptide (BNP) and N-terminal proBNP (NTproBNP)], electrocardiogram (ECG), transthoracic echocardiogram, and evaluation by a cardiologist. Patients with light chain amyloidosis were excluded.
CV assessments were performed at baseline and at the start of each chemotherapy cycle (i.e. every 3 or 4 weeks). Cardiac biomarkers were tested twice per cycle for up to 6 cycles. Patients were followed for development of CVAEs for a period of 18 months, defined according to the CTCAE v4.03: heart failure (HF), arrhythmia requiring treatment, acute coronary syndrome (ACS), grade III or IV HTN, angina, venous thromboembolic event (VTE) and pulmonary HTN (PH). All CVAEs were confirmed by a cardiologist.
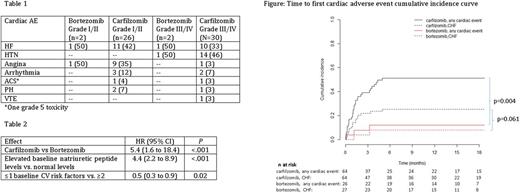
Results: 97 patients were enrolled, with 65 receiving Car- and 32 Bor-therapy with a median follow-up of 14 months. Patients received a median of 2 prior lines of therapy in the Car group, and 1 prior line in the Bor group. There was no difference in baseline natriuretic peptide levels between cohorts (P=0.26). In total, 61 cardiac AEs occurred, with 54% being ≥ grade 3 (Table 1). Of evaluable patients, 50% (n=32) receiving Car and 15% (n=4) receiving Bor experienced at least one CVAE (P=.002). The median time to CVAE from start of treatment was 33 days, with the majority (86%) occurring within the first 3 months of treatment (Figure). Seventeen patients with CVAE (47%) experienced ≥2 CVAEs.
In patients receiving Car-therapy, a baseline BNP >100 pg/ml or NTproBNP >125 pg/ml was associated with an increased risk of CVAE (OR 11.7, 95% CI 3.3-41.5; P <.001). In patients with normal baseline natriuretic peptide levels, an increase during cycle 1 was associated with a higher risk of CVAE (OR of 35.1, 95% CI 4.3-289.5, P <.001).
In multivariate analysis (Table 2), Car-therapy was associated with a higher risk of CVAE (HR 5.4, 95% CI 1.6 to 18.4; P <.001) as compared to Bor-therapy. Elevated baseline natriuretic peptide levels were associated with higher risk of CVAE (HR 4.4, 95% CI 2.2 to 8.9; P <.001), and patients with ≤ 1 baseline CVRF had a lower risk of CVAE (HR 0.5, 95% CI 0.3 to 0.9, P=0.02). Other assessments evaluated and not found to be predictive of CVAEs included baseline troponins, ECG, left ventricular ejection fraction, intravenous fluid volume, chemotherapy duration, corticosteroid use and higher Car dose levels.
The majority (68%) of patients with CVAEs resumed PI-based therapy after CVAE without chemotherapy modification; another 30% who experienced a CVAE were able to resume PI-therapy with chemotherapy modifications. Only 2% required discontinuation of therapy due to CVAE. One grade 5 toxicity occurred during Car treatment from ACS leading to sudden cardiac death.
Summary and Conclusions: This is the first prospective study designed to systematically evaluate cardiac events in patients receiving carfilzomib or bortezomib. CVAEs were more common with Car than with Bor but usually did not require discontinuation of therapy with careful management. Additionally, prospective monitoring with natriuretic peptides and detailed cardiac history to determine risk factors are useful in identifying patients at high risk of CVAEs during treatment with PIs.
Weiss: Alnylam: Honoraria; Prothena: Research Funding; Janssen: Honoraria; Prothena: Honoraria; Janssen: Research Funding. Cohen: Bristol Meyers Squibb: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Celgene: Consultancy; Janssen: Consultancy. Jagasia: Janssen: Consultancy, Research Funding; Mallinckrodt: Consultancy; Therakos: Consultancy, Research Funding. Moslehi: Pharmacyclics: Consultancy; Daiichi Sankyo: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Bristol-Myers Squibb: Consultancy; Takeda: Consultancy; Ariad: Consultancy; Acceleron: Consultancy; Incyte: Consultancy; Vertex: Consultancy; Regeneron: Consultancy; StemCentRx: Consultancy; Verastem: Consultancy; Heat Biologics: Consultancy; Rgenix: Consultancy. Savona: Sunesis: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Consultancy, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Astex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Equity Ownership; Celgene: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Vogl: Karyopharm: Consultancy; Takeda: Consultancy, Research Funding; Amgen: Consultancy; Celgene: Consultancy; Teva: Consultancy; GSK: Research Funding; Calithera: Research Funding; Constellation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal